Abstract
Introduction
Mixed chimerism (MC) after allogeneic hematopoietic cell transplantation (HCT) is associated with an increased risk of disease relapse. An unmanipulated donor lymphocyte infusion (DLI) can convert MC to full donor chimerism (FC) but can also precipitate severe acute graft-versus-host disease (GVHD). We recently showed in a phase I trial that a subset of purified CD8+CD44hi memory T cells derived from HLA-matched sibling donors may induce graft-versus-tumor reactions in patients with relapsed hematologic malignancies following allogeneic HCT with objective responses in 25% of patients and a low incidence of GVHD (Muffly et al, 2016 ASH Abstract #4615). Here we report on the preliminary results of a phase II study using CD8+ memory T-cell DLI as consolidative therapy early after transplant for patients with MC to assess the rate of conversion to FC, the risk of GVHD, and the prevention of disease relapse.
Methods
Patients: Eligible patients were 18-75 years of age with a high-risk or refractory hematologic malignancy who underwent nonmyeloablative allogeneic HCT from a matched sibling donor using total lymphoid irradiation and antithymocyte globulin conditioning, and who had MC (<95% donor CD3+ cells) at day +30. Patients were without acute GVHD at the time of the CD8+ memory T-cell DLI and were on tacrolimus or cyclosporine monotherapy for GVHD prophylaxis at the time of DLI.
CD8+ memory T cells: Phenotypic CD8+ memory T cells were isolated from matched sibling donors following two days of unmobilized apheresis using a tandem immunomagnetic selection strategy including CD45RA depletion followed by CD8+ enrichment on the CliniMACS Plus instrument. Cell purity was determined by flow cytometry analysis. CD8+ memory T cells were infused between day +30 and day +70 at a dose of 5 x 106 cells/kg, which was determined in the prior phase I trial to be the maximum dose that could be collected from two apheresis products.
Study Endpoints: Following DLI, patients were assessed for the development of acute and chronic GVHD, conversion from MC to FC (≥95% donor CD3+ chimerism) within three months, and for disease relapse. Secondary outcomes included the incidence of adverse events, invasive infections, non-relapse mortality, overall survival, and disease-free survival.
Results
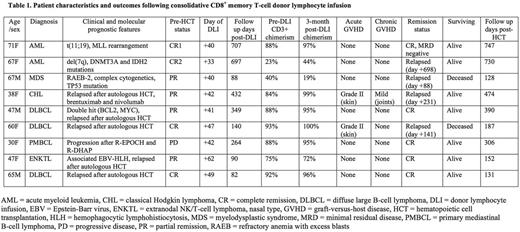
Patient characteristics and outcomes after DLI are summarized in Table 1. Nine patients received CD8+ memory T-cell DLI at a median of 42 days post-HCT (range 33-62 days). The cell product was highly enriched for CD8+/CD45RA-/CD45RO+ cells, and flow analysis confirmed the selected cells were phenotypically consistent with the CD8+ T effector memory subset with a post enrichment purity of ≥96% (range 96.4-98.8%) in all patients. Median age of the patients was 60 years (range 30-71 years) with a median follow up of 306 days post-HCT (range 131-747 days). Diagnoses included non-Hodgkin lymphoma (n=5), acute myeloid leukemia (n=2), myelodysplastic syndrome (n=1), and Hodgkin lymphoma (n=1). Median donor CD3+ chimerism at day +30 was 88% (range 23-93%).
CD8+ memory T-cell DLI was well tolerated with no adverse events. Six of the nine patients (67%) converted from MC to FC within three months post-DLI with a median donor CD3+ chimerism of 97% (range 95-100%). Two patients developed grade II acute GVHD (skin only) that was treated to resolution. One patient developed mild chronic GVHD. No patients developed severe acute GVHD (grade III-IV) or extensive chronic GVHD. No patients developed invasive infections, and non-relapse mortality was 0%. Of the three patients who failed to convert from MC to FC, two had residual disease at the time of transplant, one of whom had early disease relapse at day +88. Four of the six patients who converted to FC were in complete remission (CR) at the last follow up. One of the three patients with persistent MC was in CR at the last follow up. For the entire study cohort, the median disease-free survival is 1.1 years and the median overall survival is not yet reached.
Conclusions
Consolidative CD8+ memory T-cell DLI following nonmyeloablative matched related donor HCT is well tolerated, converted six of nine patients (67%) with MC to FC, and was associated with a low rate of GVHD with no cases of severe acute or extensive chronic GVHD in this study. Patients continue to accrue to trial, and further accrual and longer follow up are needed to determine if this strategy will reduce relapse rates for high-risk patients with MC.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal